cannetix Inc
Well-Known Member
A Research Project on The Potential Broad-Spectrum Anti-Proliferative Activity of CB1/CB2 Activation

Glucose Starvation & Oxidative Stress In Cancer Cells by Activation of CB1 and CB2 receptors

Glucose Starvation & Oxidative Stress In Cancer Cells by Activation of CB1 and CB2 receptors
Cannabinoids have long been known for their therapeutic potential when it comes to the prevention & treatment of various types of cancer. While much of the related body of research focuses on the more direct pharmacokinetics of these compounds in relation to the inhibition of various cancer-related enzymes responsible for proliferation, there is also evidence that Cannabinoids may modify how the body uptakes, metabolizes, and/or stores carbohydrates, particularly Glucose, through the activation of CB1 and CB2 Cannabinoid receptors, potentially giving Cannabinoids broad-spectrum anti-proliferative activity. Broad-spectrum treatments are of great value due to the inherent limitations of tumor-specific enzyme targeting, which make them effective only against very specific types of cancer.


https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4171598/ Cannabinoids as therapeutic agents in cancer: current status and future implications
https://meshb.nlm.nih.gov/#/record/ui?name=Cannabinoid+Receptors Cannabinoid Receptors
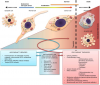
Cancer is a group of diseases characterized by abnormal, rapid cell division and tissue growth which causes death through various means depending on the particular type of cancer. In most cases, cancer kills when masses of cancerous tissue invade key organs through a process known as metastasis; the detachment of cancer cells from malignant tumors and their entry into the lymph or bloodstream. Due to the abnormal and rapid growth of cancer tissue, cancer cells have an energy demand that differs from that of healthy cells. While normal cells, under normal circumstances, use mitochondrial respiration to generate energy (unless they are deprived of Oxygen in which case they switch to anaerobic forms of metabolism), cancer cells, even in the presence of adequate oxygen, rely primarily on the process of anaerobic Glycolysis to generate energy. Anaerobic glycolysis, which converts Glucose to lactate, is characterized by high glucose consumption and high lactic acid production. In cancer, this elevated glucose consumption is referred to as glucose addiction.


http://scienceline.ucsb.edu/getkey.php?key=989 How does cancer kill a person? - USCB Science Line
https://www.ncbi.nlm.nih.gov/pubmed/26504932 Glucose Addiction in Cancer Therapy: Advances and Drawbacks
https://en.wikipedia.org/wiki/Anaerobic_glycolysis Anaerobic Glycolysis
Due to the so-called “Glucose addiction” that is universally demonstrated by cancer cells, there has been a substantial body of research focused on the stimulation or inhibition of various parts of the glucose metabolism pathway as a means of cancer therapy. These therapies, including the adjuvant use of “Ketogenic” diets, the inhibition of key tumor-specific glucose transporters (GLUTs), the inhibition of enzymes involved in glycolysis and gluconeogenesis, etc. have proven quite promising. When healthy cells are deprived of Glucose, signals are sent to the liver causing ketone bodies to flow to the extra-hepatic tissue where they are used as fuel in the metabolic process of ketolysis. Because cancer cells show reduced expression of the enzymes responsible for ketolysis, data suggest that glucose starvation & dietary ketosis leads to significantly elevated levels of oxidative stress in cancer cells. Oxidative stress is defined as an imbalance between the production of reactive oxygen species (free radicals) including Superoxide (O2-) and Hydrogen peroxide (H2O2) and antioxidant defenses including Superoxide Dismutase (SOD). This increase in reactive oxygen species stimulates pro-apoptotic responses mediating programmed cell death in cancer cells while limiting apoptosis of healthy, non-cancerous cells.


https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4867238/ Hyperketonemia and ketosis increase the risk of complications in type 1 diabetes
https://www.ncbi.nlm.nih.gov/pubmed/20009288 Glucose deprivation-induced metabolic oxidative stress and cancer therapy
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2129159/ Metabolic Effects of the Very-Low-Carbohydrate Diets
https://www.ncbi.nlm.nih.gov/pubmed/10693912 What is oxidative stress?
https://en.wikipedia.org/wiki/Superoxide_dismutase Superoxide Dismutase
https://www.ncbi.nlm.nih.gov/pubmed/17786631 Reactive oxygen species in mitochondria-mediated cell death
https://www.ncbi.nlm.nih.gov/books/NBK26873/ Programmed Cell Death (Apoptosis)
Another potentially promising glucose related therapy for cancer treatment is Insulin Potentiation Therapy. Due to the widely known fact that many, if not all cancer cells have elevated levels of Insulin receptor sites Insulin Potentiation Therapy is thought to potentiate existing cancer therapies by 1) improving the site-specific targeting of chemotherapy drugs, 2) reducing blood glucose levels by stimulating glucose uptake by muscle cells, and 3) Increasing glucose-addiction in cancer cells. One problem with Insulin Potentiation Therapy is its potential side-effects and potential for misuse. Insulin is tightly associated with the progression of cancer cells via its general effects on cell proliferation as well as (likely) its effect of promoting glucose uptake by cells (although the complex mechanisms involved in insulins role in tumor growth are not yet fully understood).


https://www.ncbi.nlm.nih.gov/pubmed/22649741 Low-dose chemotherapy with insulin (insulin potentiation therapy)
https://www.omicsonline.org/insulin...ree-year-study-1948-5956.1000117.php?aid=5970 Insulin Potentiation Therapy in the Treatment of Malignant Neoplastic Diseases: A Three Year Study
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC296896/ Elevated insulin receptor content in human breast cancer
https://www.ncbi.nlm.nih.gov/pubmed/27368923 Insulin, insulin receptors, and cancer
https://www.ncbi.nlm.nih.gov/pubmed/9575831 Insulin stimulation of glucose uptake in skeletal muscles
http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0021594 Insulin Promotes Glycogen Storage and Cell Proliferation in Primary Human Astrocytes
https://molecular-cancer.biomedcentral.com/articles/10.1186/1476-4598-12-72 Insulin enhances metabolic capacities of cancer cells by dual regulation of glycolytic enzyme pyruvate kinase M2